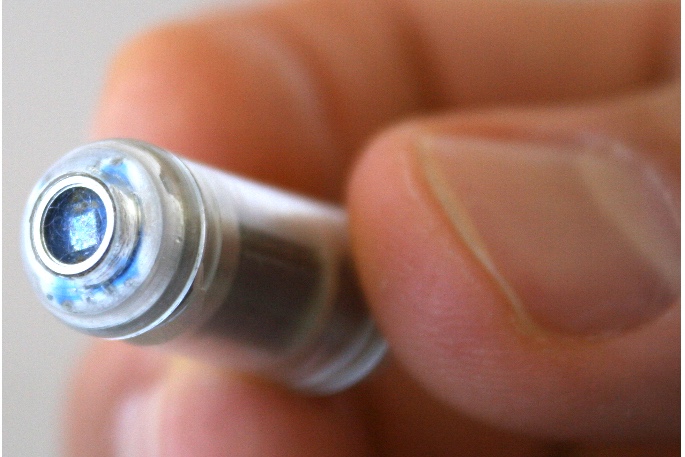
Check-Cap uses a disposable capsule that travels naturally through the digestive tract and is excreted without any intervention. X-ray technology embedded in the capsule provides a 3-D angular scan of the colon that, unlike the optics used in a scope, can see through the contents of the bowels. As the capsule passes through the colon, an external data receiver collects the images and analyzes them as part of the cancer screening process.
The technology is sensitive enough to detect clinically significant polyps, but unlike a scope, it cannot be used to remove them for biopsy. If a polyp or suspected tumor is detected, the patient must undergo a traditional colonoscopy procedure to remove the possibly diseased tissue for further analysis. Despite this drawback, the method may be beneficial for patients who require a screen due to age, but have a relatively low risk of colon cancer. This allows them to have the necessary screening procedure with minimal discomfort. It also may expand screening to patients who are hesitant to undergo a colonoscopy due to its uncomfortable preparation and the invasive nature of the procedure itself.
Check-Cap is still in the early stages of development and is not yet available for sale or clinical use. It is in the middle of clinical trials in Europe with a similar program starting soon in the US as part of the FDA approval process. When it launches, the Check-Cap capsule is expected to cost $600 per scan as compared to $1,000 to 3,000 for a colonoscopy.




